-
CANADA-EN
North America
South America
Europe
- Belgium (FR)
- Belgium (NL)
- Denmark
- Deutschland
- Europe
- España
- France
- Ireland
- Italia
- Nederland
- Norge
- Polska
- Россия
- Suomi
- Sverige
- United Kingdom
- Schweiz (FR)
- Schweiz (CH)
- Türkiye
Middle East / Africa
Asia / Pacific
|
Compatible with widely used pen injection devices1* |
|
|---|---|
|
Lilly |
KwikPen (Basaglar, Humalog, Humulin, Lyumjev, Mounjaro/Zepbound, Rezvoglar), Forteo, Tempo Pen |
|
Novo Nordisk |
FlexPen (Novolin, NovoLog), FlexTouch (Awiqli, Fiasp, Levemir, Tresiba), Ozempic, Saxenda, Victoza, Wegovy, Xultophy, NovoPen Echo |
|
Sanofi |
SoloStar (Admelog, Apidra, Lantus, Toujeo), Soliqua |
|
Biocon Sdn. Bhd. |
Kirsty |
|
Viatris |
Semglee |
|
*Listed brands are owned by third parties. Reference: 1. Compatibility Confirmation for Pen Needles/1490TH-0004-20. Rev Y – 2025-11-18. |
|
Nano PROTM Pen Needles can help you deliver insulin correctly1
Insulin injection mistakes are more common than you may think2*
A study showed that people with diabetes were making at least 1 mistake in how they inject insulin.2*
of patients apply
too much injection force2*
The most common mistake was applying too much force against the skin when injecting, which can increase the chance of injecting into the muscle—leading to pain or the chance of blood sugars dropping too low.2,3
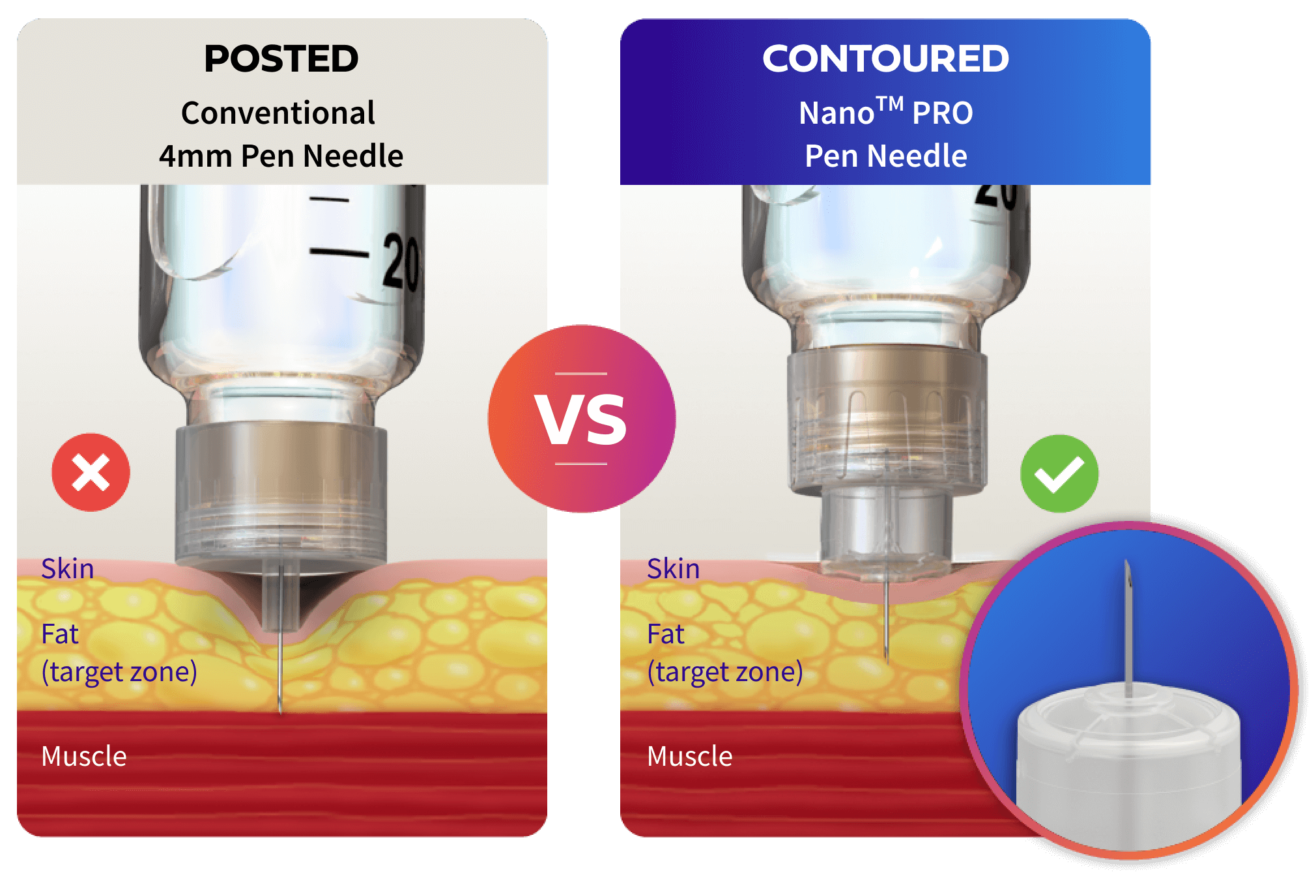
Deliver your insulin correctly with a contoured needle base
The unique contoured needle base on Nano PROTM Pen Needles helps to correct for different forces that may be applied to the skin when giving an injection to help make sure insulin gets where it needs to go.1†
Nano PROTM Pen Needles can lower the risk of injecting into your muscle by

vs other 4mm posted
base pen needles.1‡
Explore the innovative features
of the Nano PROTM Pen Needles
The combination of innovative features on Nano PROTM Pen Needles was found by people with diabetes to significantly reduce overall injection pain and make the injection experience easier compared to other pen needles studied.4§
Consistency with the contoured base
Nano PROTM Pen Needles, with a unique contoured base, allow for more consistent 4mm injections.1¶

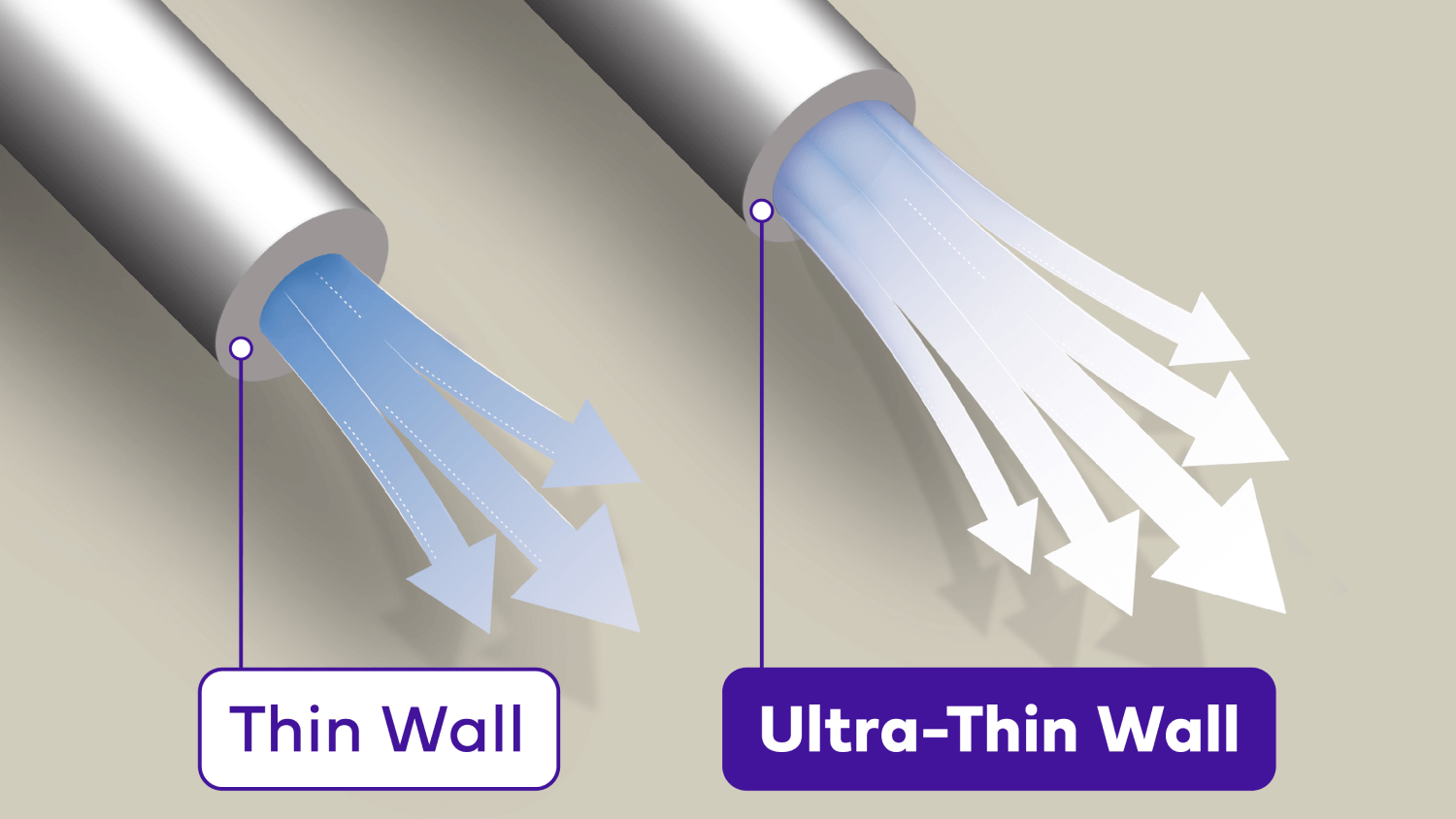
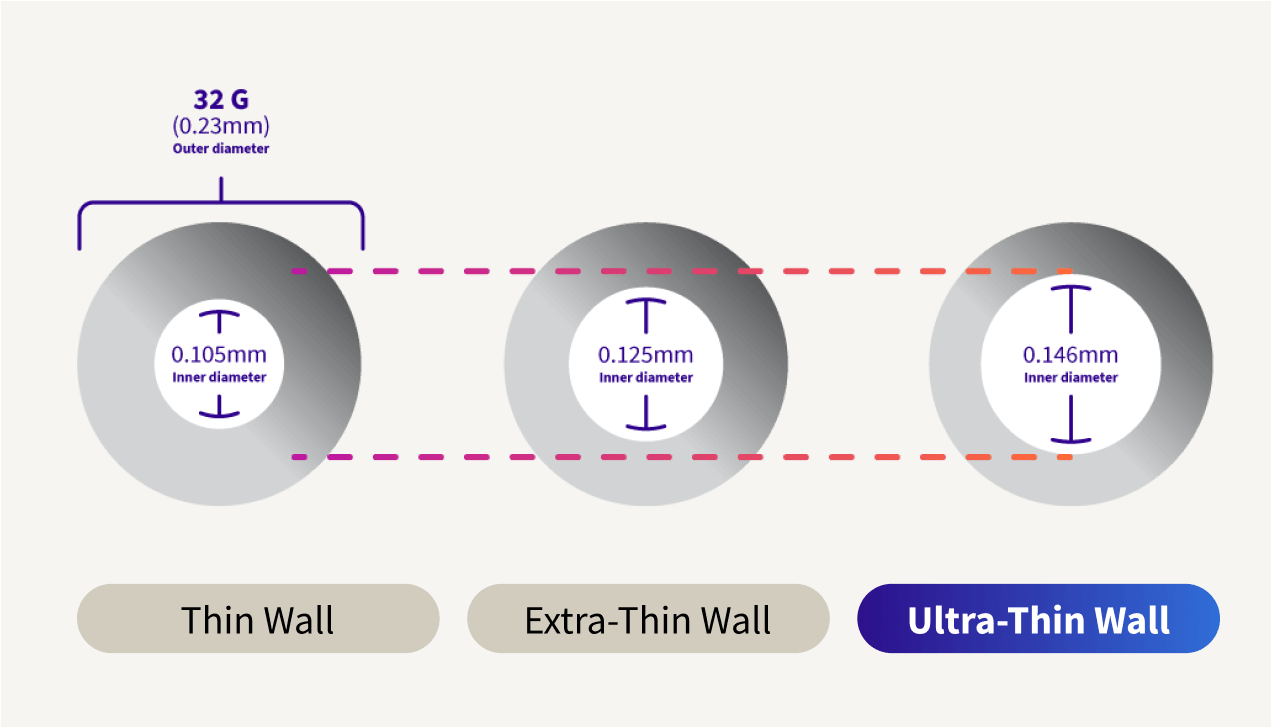
Confidence with Ultra-Thin wall
Ultra-Thin wall technology increases the flow of insulin, making injections easier and giving you the confidence that you have received the full dose.6||
Ultra-Thin wall is the highest ISO standard for a 32G pen needle inner diameter.7
Designed for ease of use
The wider outer cover is easier to attach to a pen device and the larger inner needle shield is easier to grip to remove before an injection.4§#
Nano PROTM Pen Needles are compatible with widely used pen injection devices8
See Our Compatibility Chart
Discover resources that could help you reach your treatment goals
Embecta Resources Hub*230 patients with diabetes surveyed as part of a cross-sectional observational behavioral study in Canada.
†Results from an imaging study of insulin under the skin comparing Nano PROTM Pen Needles vs other 4mm posted base pen needles.
‡To precisely locate injection depth, 1188 injections were administered in swine across a range of clinically relevant injection forces using 20µl of iodinated contrast delivered with Nano PROTM vs three 4mm posted-hub pen needles. Intramuscular injection risk was calculated through an in silico probability model, using needle penetration depth and published average human tissue thickness measurements.
§Results from clinical study assessing patient preferences and feedback.
¶Results from an imaging study of insulin under the skin comparing Nano PROTM Pen Needles vs other 4mm posted hub pen needles.
#Patients were aware of the pen needle they were using.
||Ultra-thin wall was introduced as an ISO standard for inner wall diameter in 2016.
††embecta’s use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between embecta and the owners of these trademarks.
References
1. Rini C, Roberts BC, Morel D, et al. Evaluating the impact of human factors and pen needle design on insulin pen injection. J Diabetes Sci Technol. 2019;13(3):533-545.
2. Bari B, Corbeil MA, Farooqui H, et al. Insulin injection practices in a population of Canadians with diabetes: an observational study. Diabetes Ther. 2020;11(11):2595‑2609.
3. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231‑1255.
4. Whooley S, Briskin T, Gibney MA, et al. Evaluating the user performance and experience with a re-engineered 4 mm x 32G pen needle: a randomized trial with similar length/gauge needles. Diabetes Ther. 2019;10(2):697-712.
5. Hirsch L, Gibney M, Berube J, Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J Diabetes Sci Technol. 2012;6(2):328-335.
6. Aronson R, Gibney MA, Oza K, et al. Insulin pen needles: effects of extra-thin wall needle technology on preference, confidence, and other patient ratings. Clin Ther. 2013;35(7):923-933.
7. Stainless steel needle tubing for the manufacture of medical devices—requirements and test methods. ISO 9626. 2016.
8. Compatibility Confirmation for Pen Needles/1490TH-0004-20. Rev Y – 2025-11-18.