-
UNITED KINGDOM
North America
South America
Europe
- Belgium (FR)
- Belgium (NL)
- Denmark
- Deutschland
- Europe
- España
- France
- Ireland
- Italia
- Nederland
- Norge
- Polska
- Россия
- Suomi
- Sverige
- United Kingdom
- Schweiz (FR)
- Schweiz (CH)
- Türkiye
Middle East / Africa
Asia / Pacific
You may be surprised how innovation in pen needle design can support patients in their insulin delivery experience1-2


New lookSame products you know and trust
We are excited to introduce you to the new embecta packaging.
LEARN MOREInsulin injection technique varies, leaving patients at risk for injection error3*
Despite your best effort to educate patients about proper injection technique, it has been shown that most patients inject their insulin using a wide range of techniques, which can impact the correct delivery of insulin.3*
“One of the things that Iʼm always worried about is how do I know that once I inject it into my body itʼs in the right place or itʼs actually working.”†
Patient quote from market research
Help your patients experience more comfortable and confident injections1-2
Micro-Fine UltraTM 4mm Pen Needles have innovative features proven to provide an easier, more comfortable injection experience, and confidence that the complete dose of insulin has been delivered correctly.1-2द
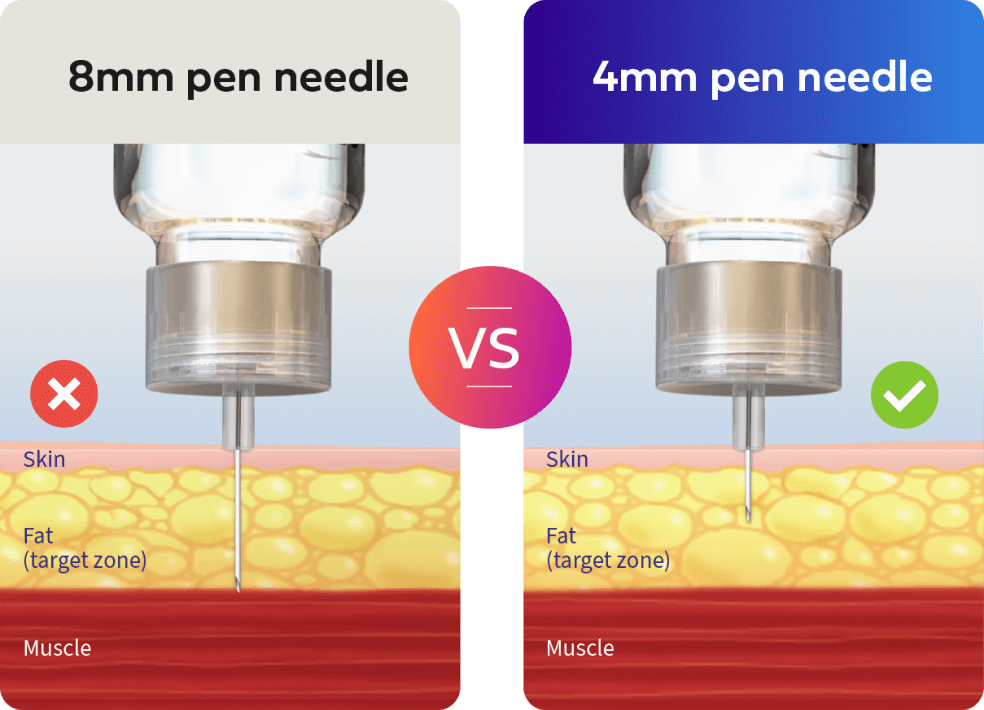
Accuracy with a 4 mm needle
An insulin needle needs to be long enough to get through the skin but short enough to not reach the muscle, as intramuscular injections have been shown to lead to greater pain and increased risk of hypoglycaemia.4
Micro-Fine UltraTM 4mm Pen Needles help reduce the risk of intramuscular injection compared to needles 6 mm and longer.5#
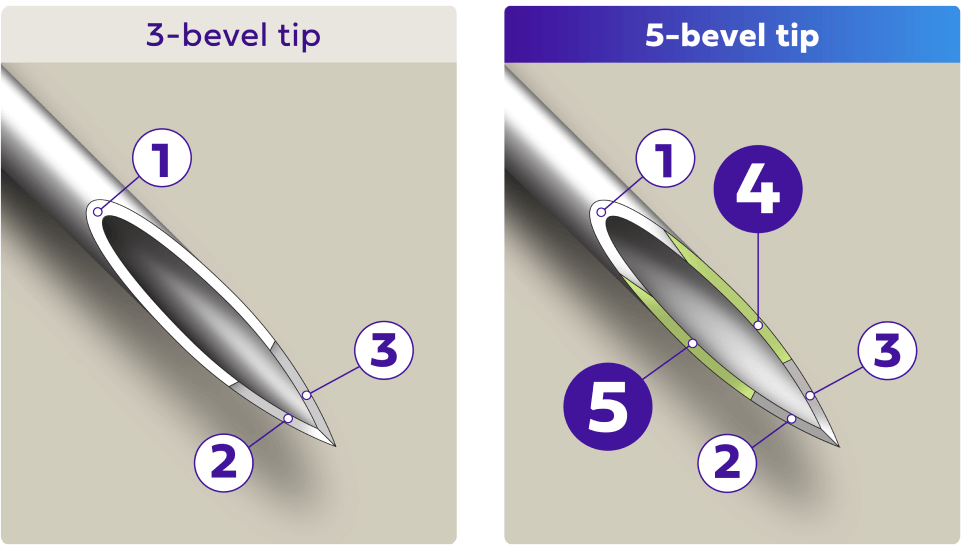
Comfort with a 5-bevel tip
A 5-bevel needle tip is designed for a more comfortable injection with less perceived injection pain compared to 3-bevel pen needles studied.2§
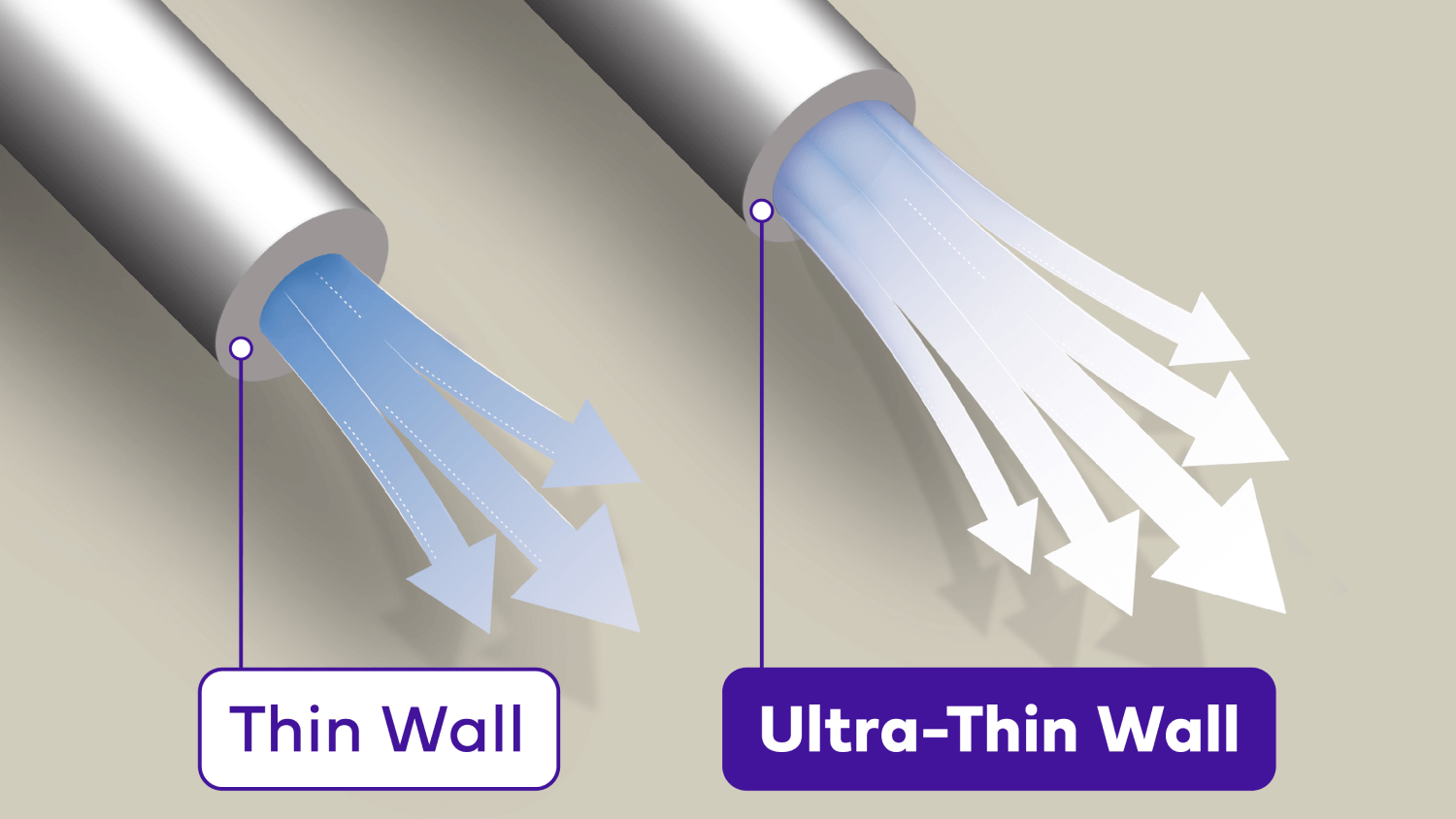
Confidence with
Ultra-Thin wall technology
Studies have shown that Micro-Fine UltraTM 4mm with Ultra-Thin wall technology increases the flow of insulin, resulting in greater ease of use and increased patient confidence that the full dose has been delivered.1‡¶
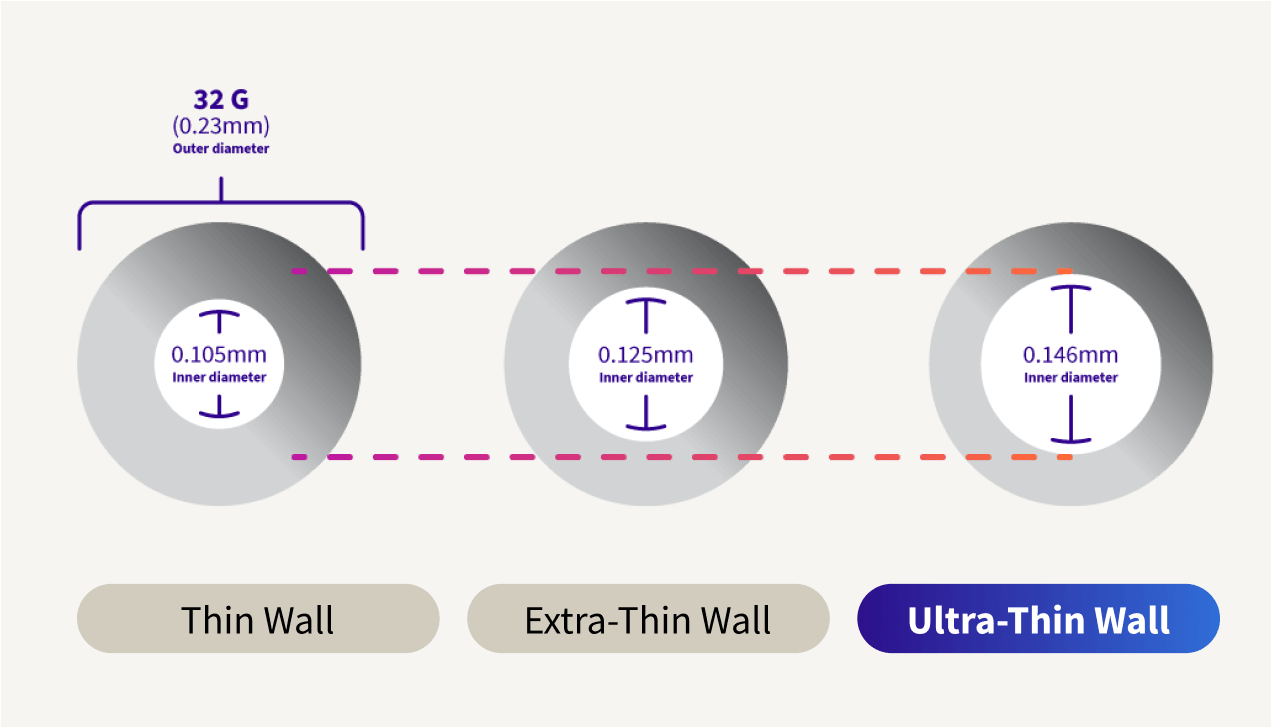
Ultra-Thin wall is the highest ISO standard for a 32G pen needle inner diameter.6
Micro-Fine UltraTM and VivaTM Pen Needles are compatible with widely used pen injectors. Even if injection therapy changes, your patients with diabetes can still use Micro-Fine UltraTM and VivaTM Needles.7
See Our Compatibility Chart
Discover resources that could help your patients reach their treatment goals
Embecta Resources Hub*N=230 patients with diabetes surveyed as part of a cross-sectional observational behavioural study in Canada.
†Patient quote from market research, August 2022.
‡198 patients with diabetes were included in this prospective, multicentre, randomised, open-label, 2-period, crossover study to evaluate differences in perceived thumb force and in confidence that the full dose of insulin was delivered, between the participants’ usual pen needle (PN) and the corresponding extra-thin wall (XTW) pen needle. Significant differences favouring XTW pen needles were seen for perceived thumb force and confidence that the full dose was delivered by 28.4 mm (95% CI, 23.7-33.2), and 24.4 mm (95% CI, 19.7-29.1), respectively; (all, P<0.001).
§86 patients with diabetes used to evaluate differences between 5-bevel and 3-bevel pen needle tips across pen needles (PN) of equal length and gauge. The 5-bevel PN would be considered more comfortable if the 95% lower bound for the percentage of insertions was greater than the 95% upper bound. After patients were informed, the 5-bevel was selected more often than the 3-bevel PN for greater comfort (p = 0.01) in home use. When patients were blinded to the PN bevel designs, no differences were found for ease of insertion (37.1%, 36.8%), comfort (37.1%, 37.6%) between the 3-vs-5-bevel PN, respectively.
¶IM risk was estimated based on 90- or 45-degree insertion. Skin surface to muscle depth was measured by ultrasound. No actual injections were performed.
#Ultra-thin wall was introduced as an ISO standard for inner wall diameter in 2016.
References
1. Aronson R, Gibney MA, Oza K, et al. Insulin Pen Needles: Effects of Extra-Thin Wall Needle Technology on Preference, Confidence, and Other Patient Ratings. Clin Ther. 2013;35(7):923-933.
2. Hirsch L, Gibney M, Berube J, Manocchio J. Impact of a Modified Needle Tip Geometry on Penetration Force as well as Acceptability, Preference, and Perceived Pain in Subjects with Diabetes. J Diabetes Sci Technol. 2012;6(2):328-335.
3. Bari B, Corbeil MA, Farooqui H, et al. Insulin Injection Practices in a Population of Canadians with Diabetes: An Observational Study. Diabetes Ther. 2020;11(11):2595-2609.
4. Frid AH, Kreugel G, Grassi G, et al. New Insulin Delivery Recommendations. Mayo Clin Proc. 2016;91(9):1231-1255.
5. Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519-30.
6. Stainless steel needle tubing for the manufacture of medical devices --requirements and test methods. ISO 9626. 2016.
7. Pen Needle Compatibility Status Summary with Diabetes Care & Non-Diabetes Drug Delivery Devices - Document Number: 149OTH-0004-20 Rev Y – 2025-11-18.
These devices are UKCA-marked in accordance with UK Medical Device Regulations.
|
Compatible with widely used pen injection devices1* |
|
|---|---|
|
Lilly |
HumaPen (Ergo II, Luxura, Luxura HD, Savvio), KwikPen (Abasaglar, Humalog, Humulin, Huminsulin, Lyumjev, Liprolog, Mounjaro/Zepbound), Forsteo, HumatroPen, Tempo Pen |
|
Novo Nordisk |
FlexPen (Insulatard, NovoMix, NovoRapid), FlexTouch (Fiasp, Levemir, Tresiba), Ozempic, Saxenda, Victoza,Wegovy, Xultophy, NovoPen 4,5,6, NovoPen Echo, NovoPen Echo Plus, Novo Pen Junior |
|
Sanofi |
DoubleStar (Toujeo), SoloStar (Admelog, Apidra, Insulin Aspart, Lantus, Lispro, Toujeo, Trurapi), Lyxumia, Soliqua, AllSTAR, AllSTAR PRO, ClikSTAR, JuniorSTAR, TactiPen |
|
Medtronic |
InPen |
|
Sandoz |
SurePal |
|
Viatris |
Semglee |
|
*Listed brands are owned by third parties. Reference: 1. Pen Needle Compatibility Status Summary with Diabetes Care & Non-Diabetes Drug Delivery Devices. Document Number: 149OTH-0004-20 Rev Y – 2025-11-18. |
|